Abstract
Introduction Cardiac inotrope medications administered to cardiac surgical patients carry steep risk–benefit trade-offs, yet wide inter-institutional variation exists in inotrope practices. Despite known wide variation in use of any inotrope for cardiac surgery, limited multicentre data exist regarding determinants of inotrope selection and time course for use. Additionally, the reasons that underpin how clinicians decide on inotrope usage and the factors that influence inotrope practice change are not well understood.
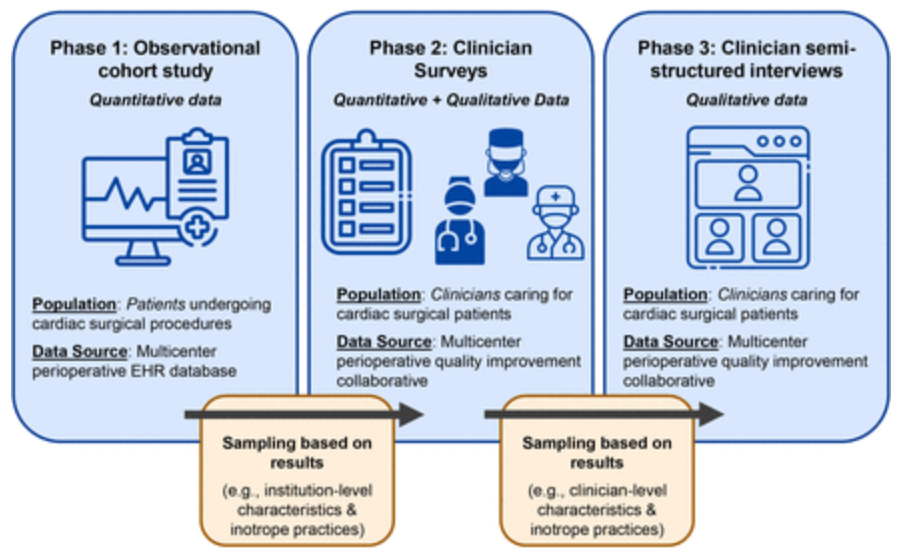
Methods and analysis This is an investigator-initiated, multicentre mixed methods study. Quantitative data will include electronic health records from an observational cohort of adult cardiac procedures within the Multicenter Perioperative Outcomes Group (MPOG) database, comprising cardiac surgical procedures from over 30 US academic and community hospitals. Additional quantitative data will be collected via surveys of clinicians involved in inotrope decision-making, contacted through an existing multicentre research and quality improvement infrastructure with engaged clinician representatives participating across MPOG hospitals. Qualitative data will be collected from open-ended questions within surveys, as well as semi-structured interviews with surveyed clinicians, sampled across approximately six institutions selected for diversity of settings and inotrope practices. An explanatory sequential mixed methods design will merge quantitative and qualitative data to develop meta-inferences explaining inotrope practices, as guided by an existing framework for characterising clinical practice variation and levers for practice change.
Ethics and dissemination The study is approved by the institutional review board at the University of Michigan Medical School (HUM00245353). Findings will be disseminated through peer-reviewed journals, conference proceedings and quality improvement forums. The study began in February 2025 and will continue until 2028.